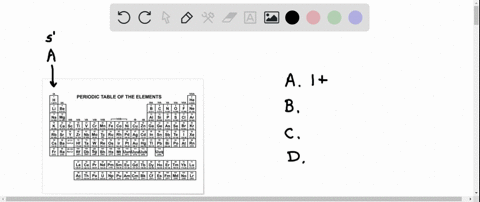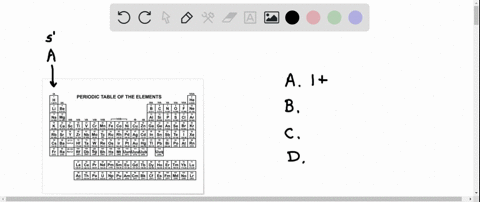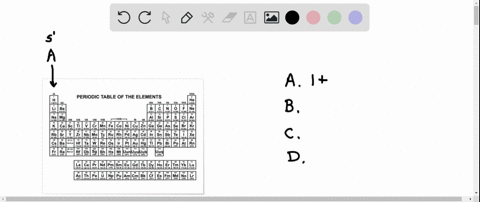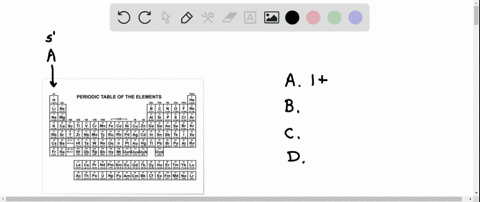
Enter the Ions Present in a Solution of K3po4k3po4.
Since K is in group 1 of the periodic table it loses one electron to form ion ie K1. What is H in the solution after 30 mL of 0370 M.
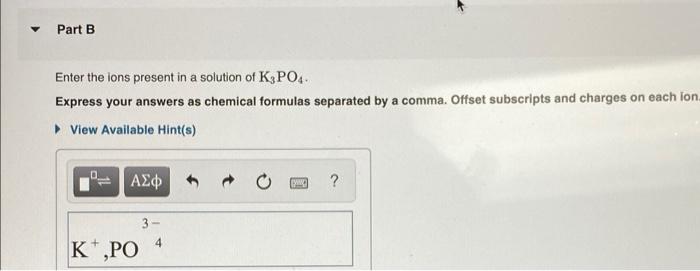
Solved Part B Enter The Ions Present In A Solution Of K3po4 Chegg Com
K CO3 2-.

. The balanced dissociation for for K3PO4 is. Write the ions present in a solution of na3po4. The ions that are present in the solution of sodium phosphate is the sodium ions and the phosphate ions.
The ions present in the solution of Na₃PO₄ are. See the answer See the answer done loading. Enter the chemical symbol of the element.
The compound is potassium trioxocarbonate IV. NaOH has been added. So 1 mole of NaCl weighs 585g.
32 moles xx 6022 xx 1023 unitsmole 193 xx 1024 ions. Trioxocarbonate IV ion has a charge of 2-and so the ions of the compound are as shown in the answer above. Cmon you guys.
Write the ions present in a solution of K3PO4Express your answers as chemical formulas separated by a comma. And 1 phosphate ion PO₄³ is present this is anion - When these cations and ions meet together a compound is formed in this case 3 sodium ions make a bond with 3 oxygens of phosphate and makes a compound of sodium. Determine whether each compound is soluble or insoluble.
Part B Which substance is the oxidizing agent in this reaction. 100 10 ratings Transcribed image text. There are 3 sodium ions Na¹ are present these are cations.
This means that in 1dm3 of solution there must be 1 mole of sodium ions. 68 10 6 M b. It contains cation potassium ion and acid radical trioxocarbonate IV ion.
The cation is Na it has a charge of 1 and there are three of them. 10 10 7 M e. This is an ionic compound which contains potassium ions and carbonate ions.
Enter your friends email addresses to invite them. Offset subscripts and charges on each ion. The dissociation of 1 mole of K3PO4.
Na3PO4 3Na PO4-3. The number of particles in 1 mole is given by the Avogadro Constant which is equal to 602x1023mol1. K 2 CO 3 means 2 x K and 1 x CO 3.
Therefore a solution that conducts electricity well. This problem has been solved. How many bromide ions are present in 655 mL of 0210 M GaBr 3 solution.
Enter the ions present in a solution of K2CO3. The correct formula is K 3 PO 43-. Potassium is a group 1 metal with electron arrangement 2881 and will form an ion K with arrangement 288.
You dont give any details of the solution so I hope this is enough to help you. Offset subscripts and charges on each ion. The anion is phosphate PO4-3 and it has a charge of -3.
A set of empirical rules used to determine whether an ionic compound is soluble. For NaCl this will be 23 355 585. Write the ions present in a solution of Na2CO3.
So now we need to convert grams into moles by dividing mass in grams by the mass of 1 mole. When it is in the solution it forms ions. Express your answers as chemical formulas separated by a comma.
Carbonate is an ion of formula CO 3 2-It contains 1 atom of carbon 3 atoms of oxygen and has 2 negative charges because it has. A homogeneous mixtures of a substance with water. A NaC 2 H 3 O 2 soluble NaC 2 H 3 O 2 aq.
K_3PO_4 - 3K PO_4 Therefore we have a total of 080 xx 4 32 moles of ions. Aqueous solutions and solubility. 15 10 9 M d.
A solution containing a solute that dissociates into ions. 1 See answer. Using Molarity of compounds and L of solutions moles of ions can be calculated.
The simple ones such as Na are isoelectronic with the nearest noble gas. The sodium ion has 3 charge while the phosphate ion has the -3 charge. K3PO4 --- 3K PO4-3.
The amount of potassium phosphate in the solution is 127 M 0343 L 043561 molesThe chemical formula of potassium phosphate is K3PO4 so there is three times as many moles of potassium as. Express your answers as chemical formulas separated by a comma. 0 of 8 completed.
This means that in each formula unit there are 3 potassium ions and 1 phosphate ion. Therefore 4242123 02moles 02mol025L 080M This splits into four ions. Up to 24 cash back identify the ions present in solution.
What do noble gases have to do with ions. Express your answers as chemical formulas separated by a.
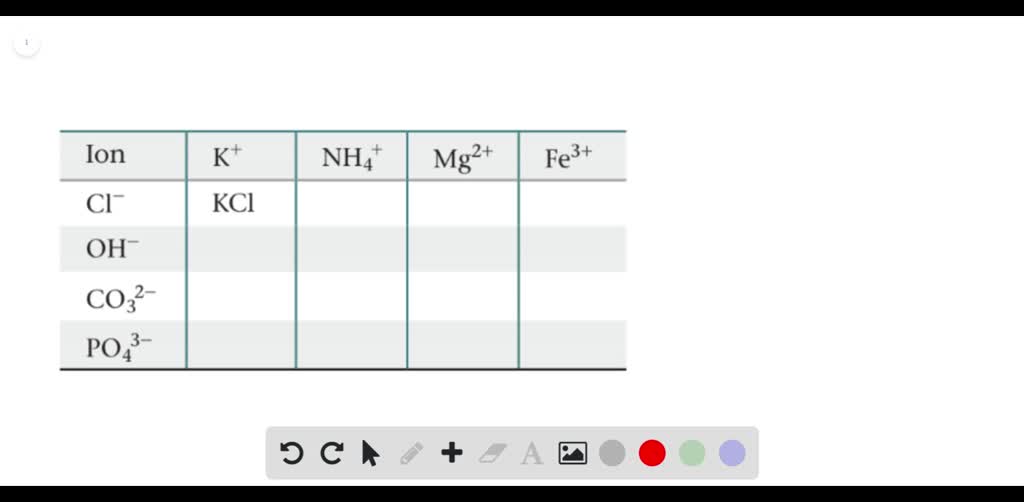
Solved Enter Ions Present In A Solution Of K3po4 Express Answers As Chemical Formulas Separated By A Comma Offset Subscripts And Charges On Each Ion
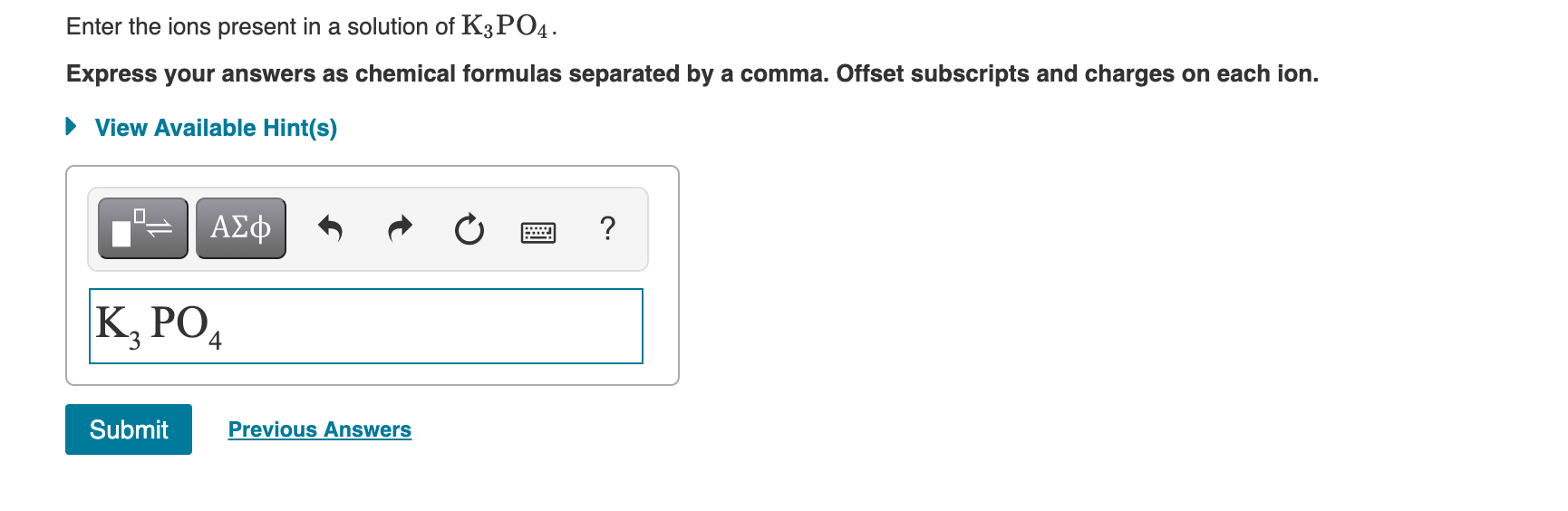
Solved Enter The Ions Present In A Solution Of K3po4 Chegg Com
Solved Enter Ions Present In A Solution Of K3po4 Express Answers As Chemical Formulas Separated By A Comma Offset Subscripts And Charges On Each Ion

Solved Enter Ions Present In A Solution Of K3po4 Express Answers As Chemical Formulas Separated By A Comma Offset Subscripts And Charges On Each Ion
No comments for "Enter the Ions Present in a Solution of K3po4k3po4."
Post a Comment